How it all started
Origin Story
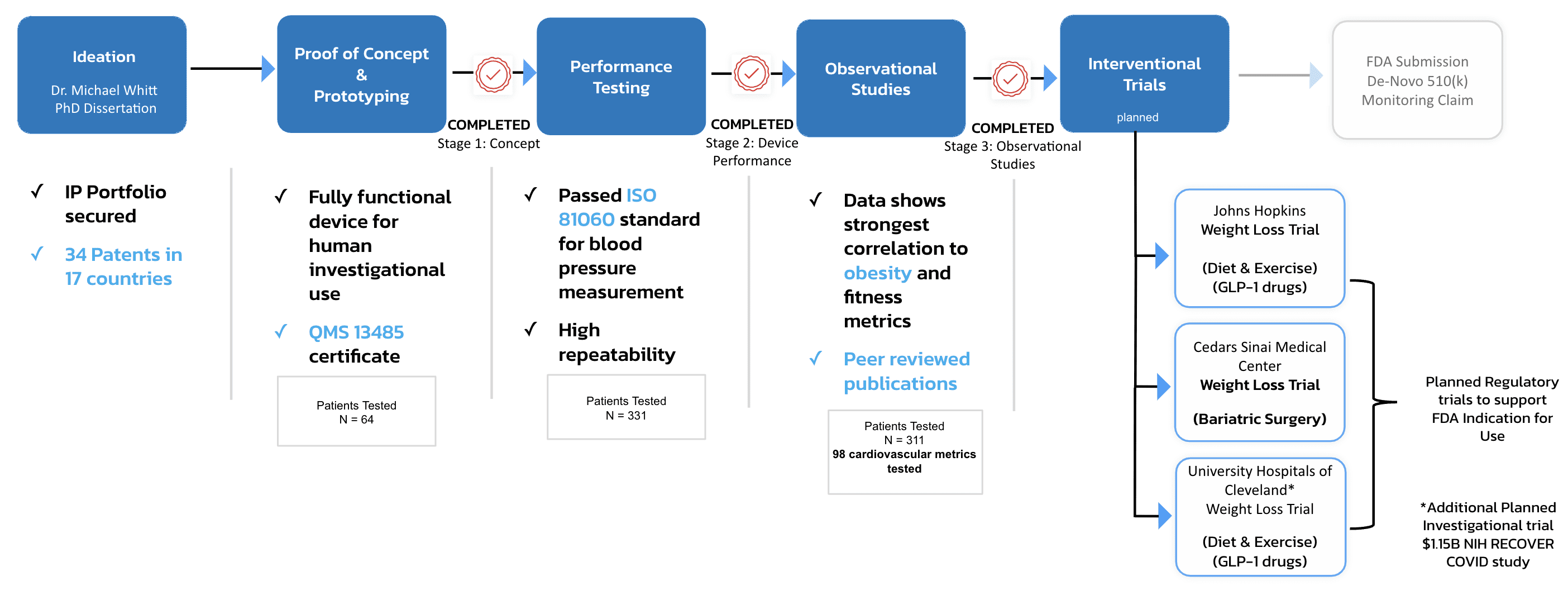
Serendipity. It was the chance meeting between a cardiac surgeon and a PhD bioengineer on the first day of class at the UCLA Anderson EMBA program. One had the idea (from his dissertation) to take the most commonly used tool in healthcare, a blood pressure cuff, and repurpose it into a linear sensor to measure the earliest warning sign of heart disease. The other understood the power and application of such a tool because she battled heart disease from inside an OR every day.
They teamed up on a class project that tasked them to create a business plan for a fictional product and pitch it to real VC’s. Instead, they pitched the non-fictional SmartCuff and the presentation was so successful that a company was born.